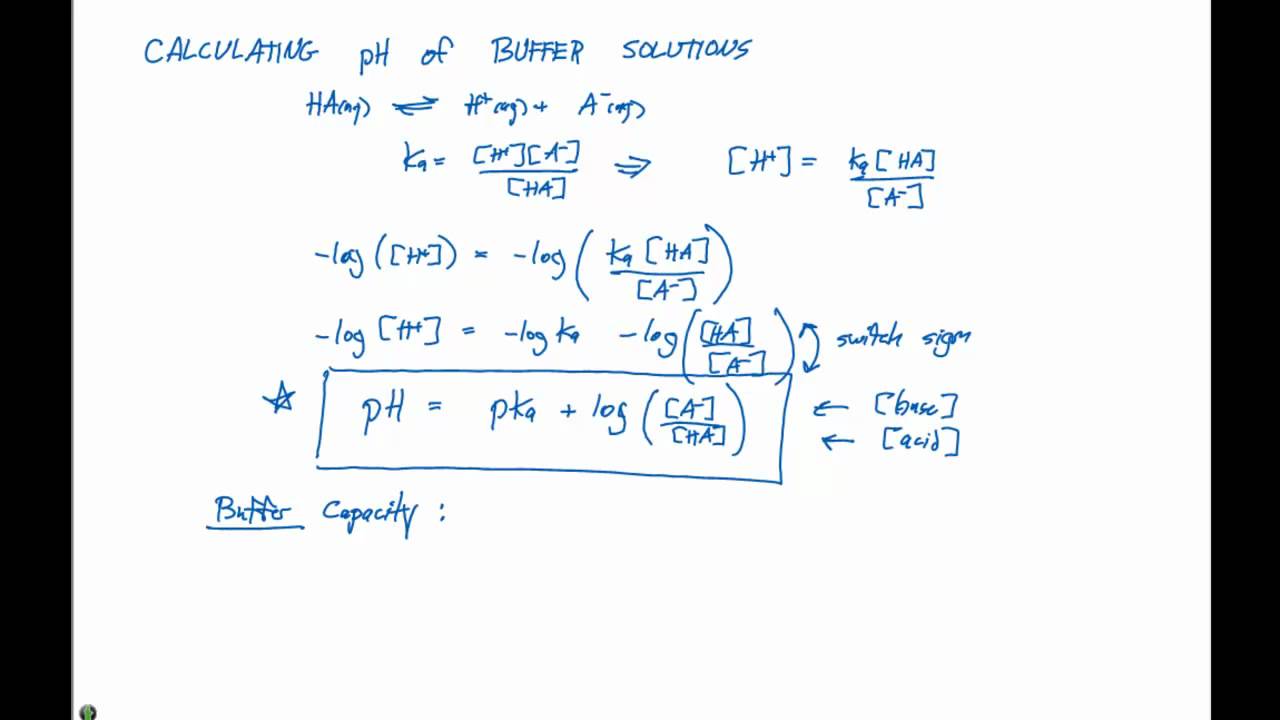
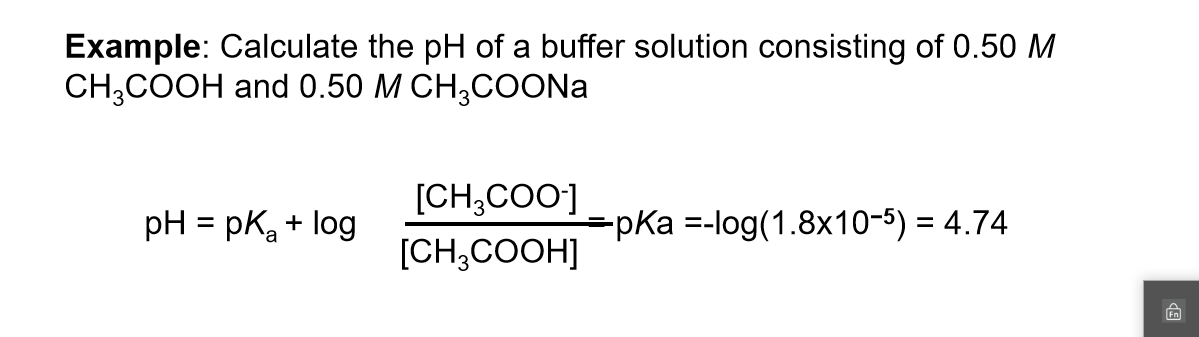
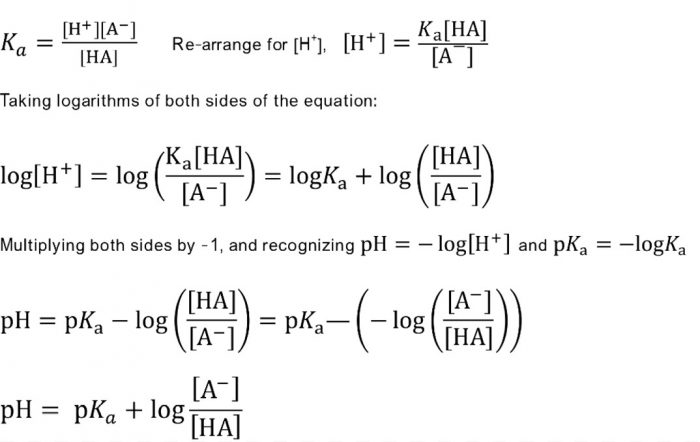
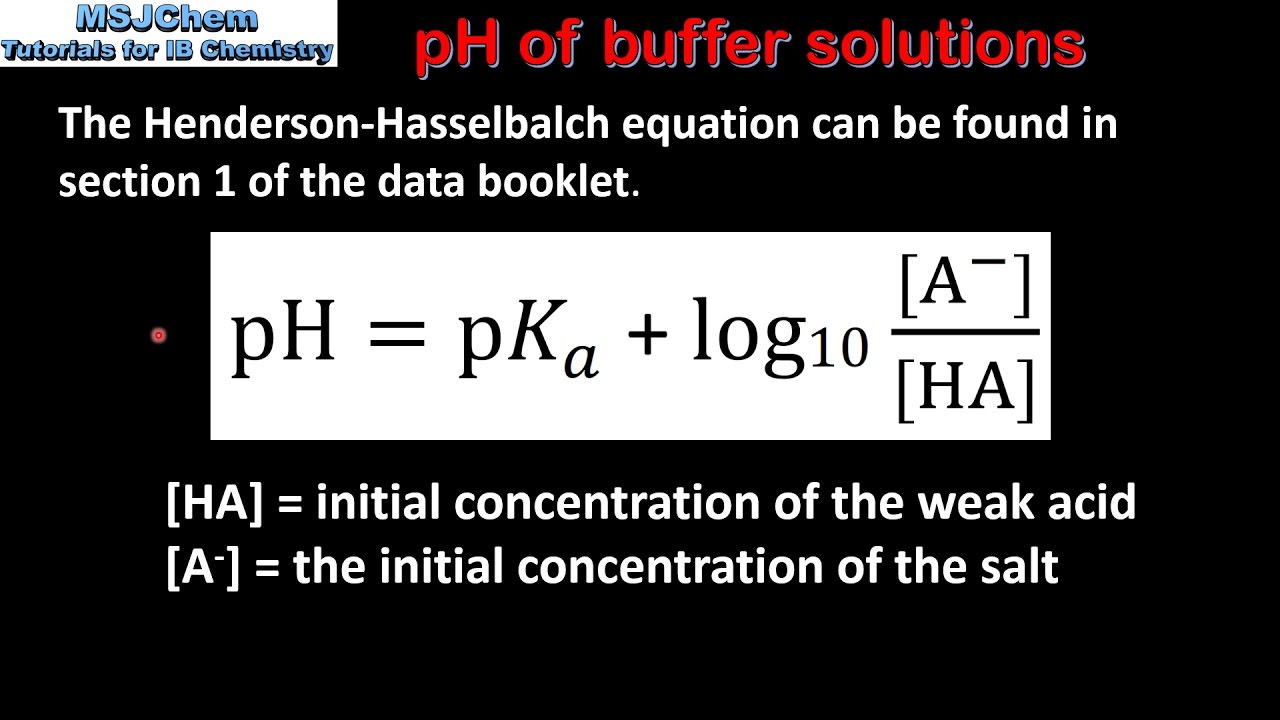
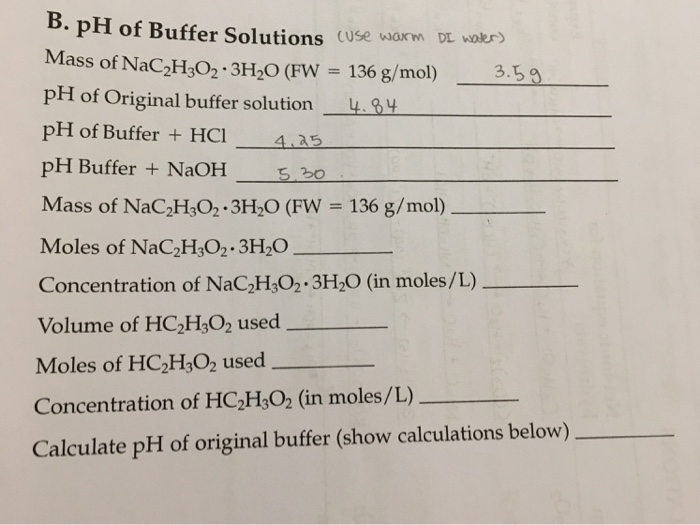
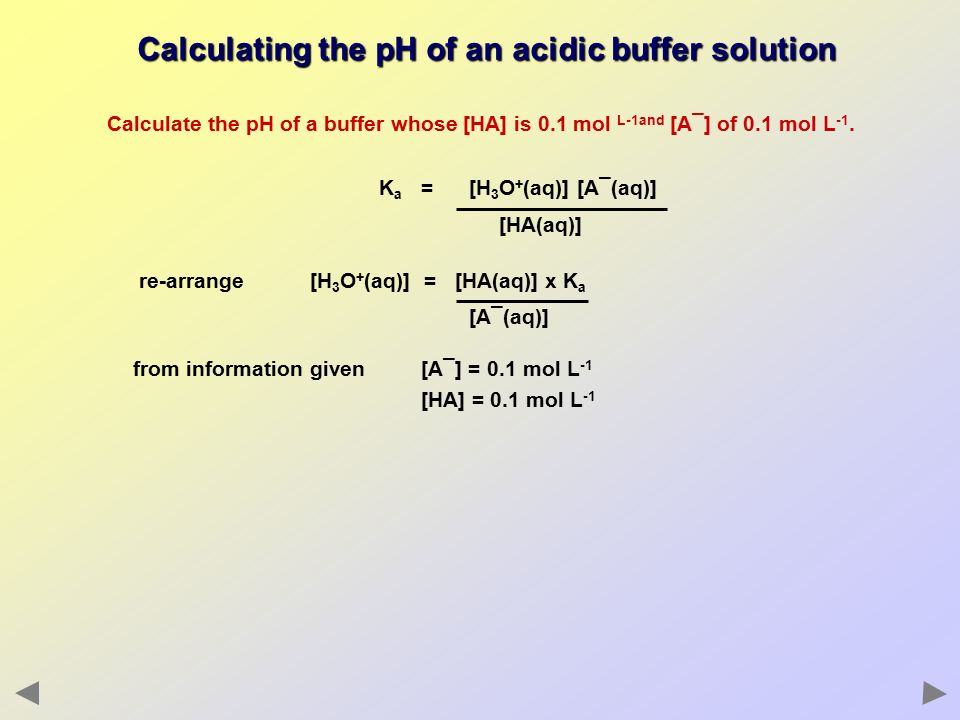
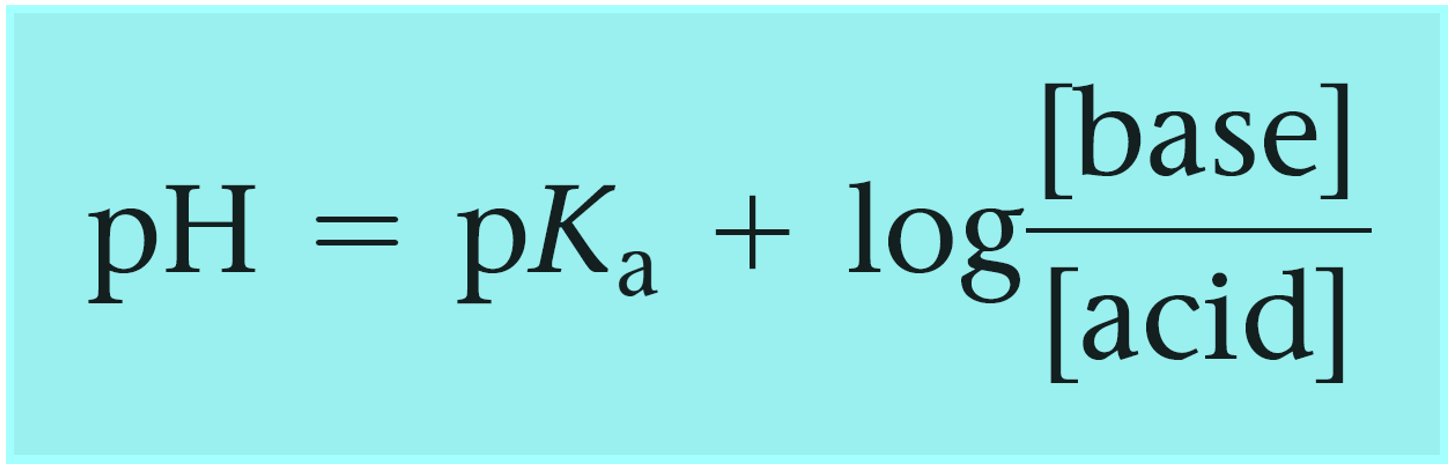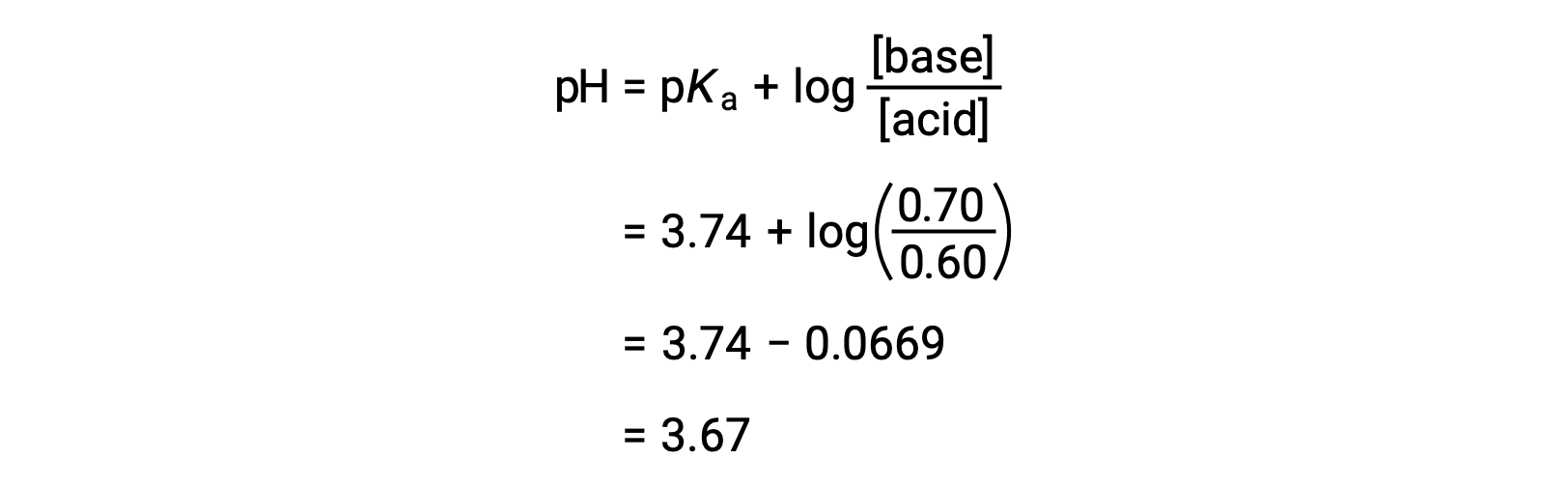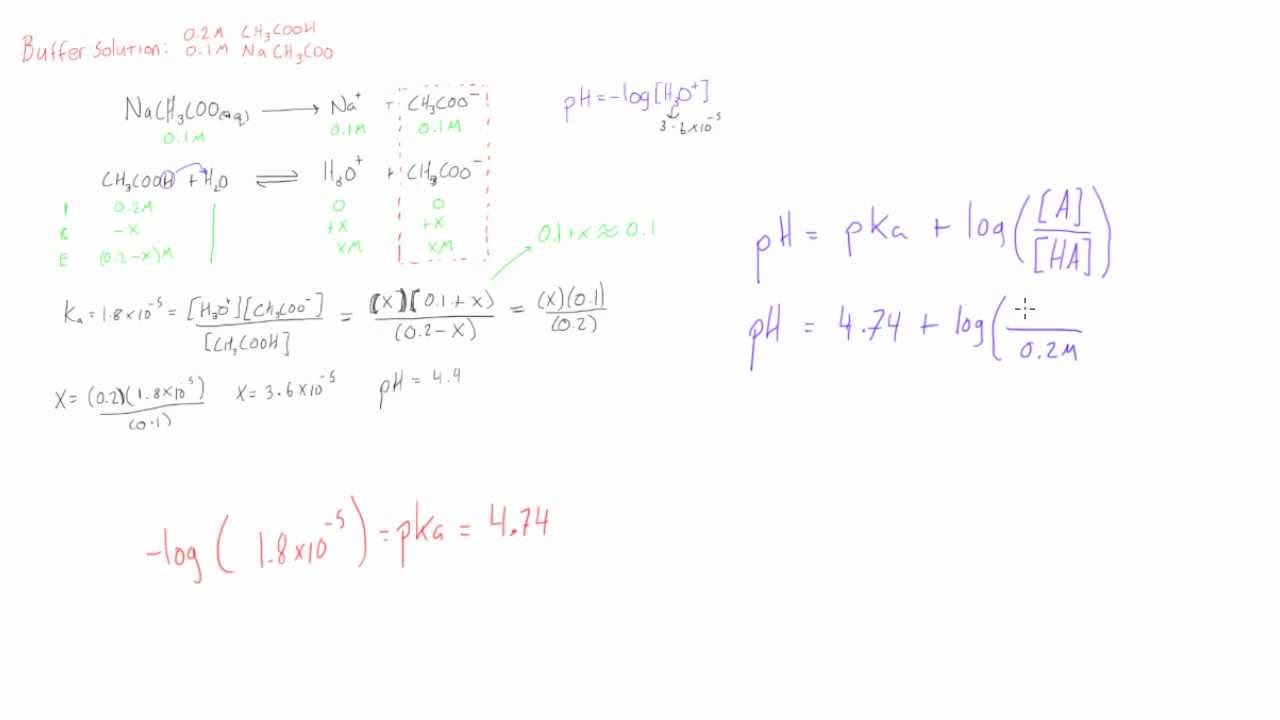Define pH. What is buffer solution? Derive Henderson-Hasselbalch equation for calculating the pH of an acid buffer solution.

SOLVED: Calculate the pH of a buffer solution made from 0.30 M hydrofluoric acid and 0.70 M sodium fluoride after the addition of 0.080 mol ofNaOHto ofthis solution Assume change in volume

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com

OneClass: Calculating pH Change For a Buffer Calculate the pH after 1.0 g of Mg(OH)_2 is added to 155...

PPT – Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer system. What is the pH after the addition of 20.0 mL of 0.050 M NaOH to 80.0 mL of